After having been diagnosed with ulcerative colitis (UC) or Crohn’s disease, the path patients with inflammatory bowel disease (IBD) and their families take is hard. Accepting the news they have received, attending all the consultations with specialists, performing the relevant tests to understand the scale of the disease or the time it takes to adjust and establish the correct treatment, are just some of the obstacles and challenges they must overcome. Indeed, one of the most complex points that the patient has to face is to succeed in receiving appropriate treatment.
News
Granulocyte-monocyte apheresis in patients with ulcerative colitis
January 10, 2023
Adacolumn

Focusing on the case of ulcerative colitis, a condition that causes inflammation in the rectum and the colon, the patient suffers relapses with more or less intense activity flare-ups, which are more or less long-lasting. The current drugs in the healthcare professional’s therapeutic arsenal for treating patients aim to induce remission of these flare-ups and keep IBD inactive to improve the patient’s quality of life. With the best efficacy and safety possible, of course.
Taking into account that some patients do not respond to conventional treatments or cannot use them due to their profile, healthcare professionals have seen granulocyte-monocyte apheresis (GMA) as a therapeutic ally offering proven benefits in patients with ulcerative colitis. This happens because the technique reduces the amount of existing inflammatory cells in the bloodstream without giving the patient anything.
What is granulocyte-monocyte apheresis?
As commented above, GMA is a non-pharmacological treatment whose basis is to pass the patient’s blood via an extracorporeal device to remove from it those pathogenic inflammatory components that determine or perpetuate the disease, thereby contributing to their treatment.
One of the granulocyte-monocyte apheresis systems sold in Europe is a system comprising a column or filter with cellulose acetate beads that enable the selective adsorption of 65% of granulocytes, 55% of monocytes/macrophages and 2% of lymphocytes, removing them from the bloodstream.
The procedure involves taking blood from the patient’s cubital vein, which flows through the circuit and the adsorption column where it is purified, and is re-infused via the contralateral cubital vein (fig. 1, we include the image of the process).
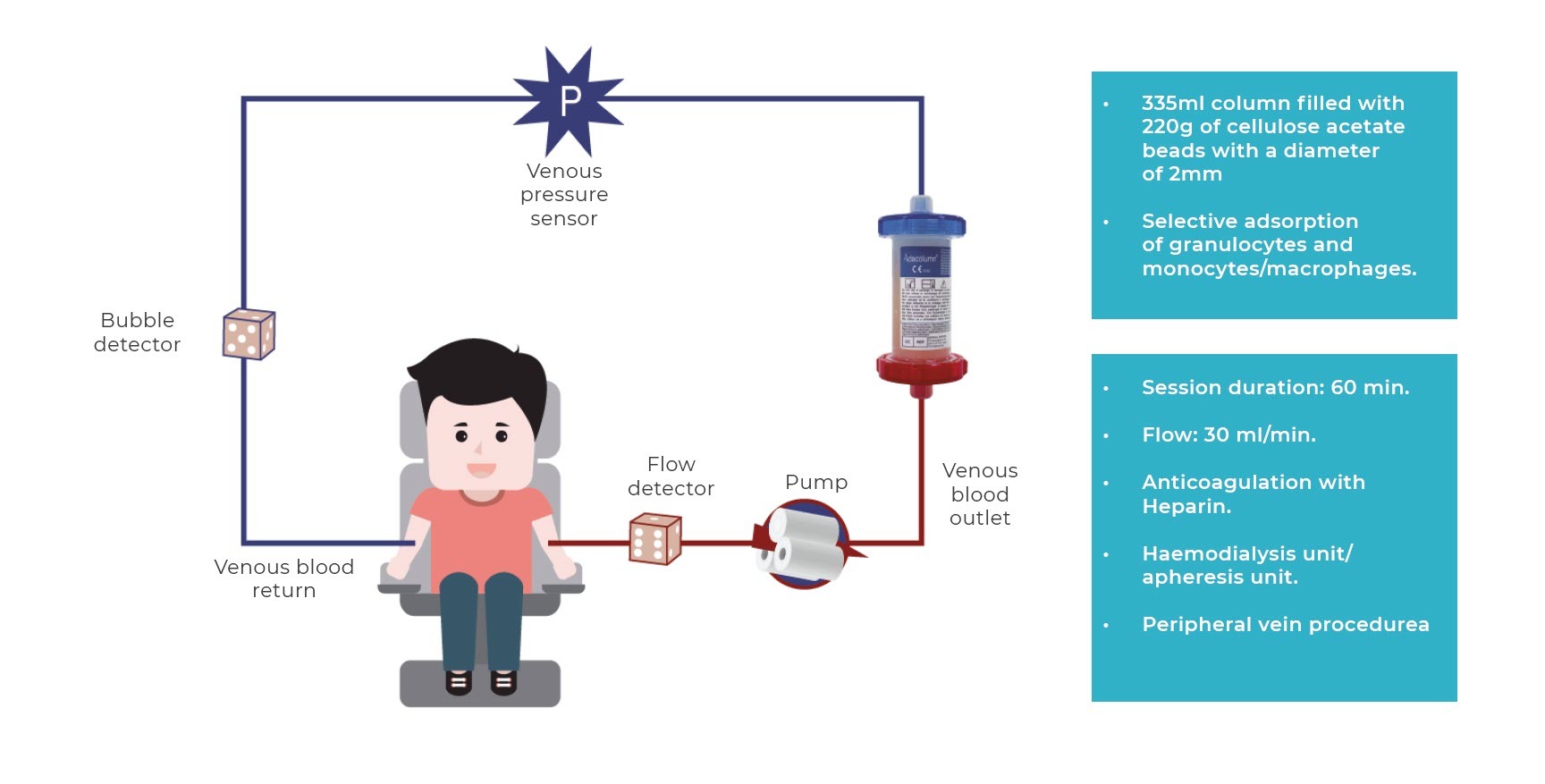
The procedure is performed in haemodialysis or apheresis units, although it can be performed in treatment units at day clinics after suitable training. The regimen for applying the technique (frequency and number of sessions) ranges from 5 to 10 sessions (1 or 2 a week) of 60 minutes or more, although actual clinical practice in the majority of hospitals involve an induction of 10 sessions (2 sessions/week for the first 3 weeks to accelerate response and then 1 weekly session until completing 10) and for a longer duration (normally 90 minutes), as this regimen has been observed to be safe and well-tolerated, obtaining the best clinical results.
Nevertheless, the only practical limitation to performing it is poor accessibility for inserting peripheral lines, which may require inserting a central line.
GMA’s mechanism of action is currently still not completely known. However, it is known that peripheral blood granulocytes and monocytes/macrophages are elevated and activated in many autoimmune diseases, whose numbers are linked to the activity and severity of the disease, and which play a key role in the disease immunopathogenesis while producing larger amounts of pro-inflammatory cytokines that are responsible for the tissue damage.
It is believed that GMA’s main adsorption mechanism is linked to the activation of the complement system’s cascade and the induction of granulocyte adhesion to the column.
Leukocytes that express Fc gamma receptor (FcγR) and complement receptors (Mac-1, CD11B/CD18) adhere to the cellulose acetate beads through activation of the complement. Thus, the decreased reservoir of activated granulocytes in the circulatory system is compensated by the mobilisation of young inactive granulocytes (CD10-) from the bone marrow to the bloodstream, without the ability to migrate to the inflammatory focal point.
Likewise, a functional change occurs in the activated monocytes, which express CD14+CD16 markers, which altogether leads to a reduction in the production of pro-inflammatory cytokines and an increase in circulating anti-inflammatory mediators; in summary, a phenomenon of immunomodulation and, accordingly, a reduction in inflammatory infiltrate and tissue damage. The direct consequence of removing leukocytes from the circulatory system is the mobilisation of young inactive leukocytes from the bone marrow, which cannot migrate to the inflammatory focal point. What is more, passing the blood through the column, in addition to decreasing the total number of inflammatory cells in the blood, also causes changes onto the surface of those that return to the body leading to a series of immunological changes that enhance the activation of previously inhibited anti-inflammatory mechanisms, initiating the resolution of inflammation. By doing so, in addition to producing immunomodulation (a change in the immune system), it reduces inflammation and tissue (cell) damage in the intestinal mucosa.
GMA is indicated for use in which patients?
There is currently a very broad therapeutic arsenal for treating IBD even though for a number of patients, these medications are ineffective or have adverse events2. Other therapeutic options are therefore needed to monitor the progression of the disease and avoid surgical procedures.
In certain scenarios, GMA is an alternative for treating this disease. This technique can be applied to different situations, including patients that do not respond well to conventional pharmacological treatments, or have a corticosteroid-dependent course, preventing relapse and in whom immunosuppressive or biological treatment is considered3,4.
Despite seeking the best treatment for each case, there is a high percentage of patients with ulcerative colitis that do not respond to conventional treatments,either those based on corticosteroids or immunomodulators (IMM thiopurines) (Ardizzone et al, 2006) or anti-TNF therapy. Until a few years ago, when a healthcare professional was in this situation where drugs failed to improve the patient’s condition, the most used option for treating them and improving their quality of life was surgery.
However, in recent decades, other treatment alternatives for patients with ulcerative colitis have emerged, like GMA, which are safer and can be applied to the following patients1:
- Adult patients with corticosteroid-dependent ulcerative colitis after failure, intolerance or elevated risk of immunomodulatory treatments and/or biologics2.
- Elderly patients with comorbidities.
- Pregnant patients.
- Paediatric patients with ulcerative colitis.
The contraindications are:
- Granulocyte levels below 2000/ML
The warnings are:
- Heparin allergy and heparin-induced thrombocytopenia
- Severe anaemia (Hb < 8 g/dL)
- Severe coagulopathy
- Active infection
- Severe heart or kidney disease
- Precautions:
- Care in situations of hypercoagulability (fibrinogen > 700 mg.mL) or dehydration (diarrhoea or recent fevers). These factors need to be corrected beforehand.
GMA, significance of efficacy and safety in UC treatment
If there are two features that define GMA, they are its safety and efficacy. As stated by Prodiggest Project3, this therapeutic option has been the subject of studies andmeta-analysis that describe it as an effective and safe alternative for patients.
On the one hand, GMA is described as being very safe for patients with ulcerative colitis because the adverse events of conventional treatments disappear. In this case, the majority of side effects are mild and temporary allowing the patient to continue with the treatment. Notably among them are headaches, chills, low-grade fever, dizziness, fatigue, myalgia, palpitations, hypotension or redness. In many case, the adverse events are linked more to the apheresis technique than to GMA treatment itself (issues with the lines etc), hence the importance of being performed by skilled professionals.
On the other hand, in addition to its efficacy in paediatric, pregnant or elderly patients, or corticosteroid-dependent and corticosteroid-refractory patients, there are other scenarios where GMA is effective. For example, it has proven that it can be a first-line treatment in distal ulcerative colitis with mild-moderate activity with a remission induction rate of 70% (Yamamoto et al 2004) and up to 80% in patients whose disease is less developed, steroid-naive (Takemoto et al 2007), as stated in Prodiggest Project.
Furthermore, it is an option that can prevent relapse in certain patients at high risk of having recurrent and difficult to control flare-ups. Accordingly, a mean duration of clinical remission obtained with GMA of 10 months can be established in patients with ulcerative colitis (Ljung et al 2007), and can even be much longer than a year the earlier the disease or the less treatment the patient has received prior to GMA (corticoids, thiopurines, biologics, etc.).
- https://www.aegastro.es/publicaciones/publicaciones-aeg/protocolos-asistenciasles-prodiggest/uso-racional-de-la-granulocitomonoaferesis-en-la-enfermedad-inflamatoria-intestinal
- Adacolumn® – Adacyte
- https://www.aegastro.es/publicaciones/publicaciones-aeg/protocolos-asistenciasles-prodiggest/uso-racional-de-la-granulocitomonoaferesis-en-la-enfermedad-inflamatoria-intestinal
- Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, AllezM, et al. Second European evidence-based consensus on thediagnosis and management of ulcerative colitis part 2: Currentmanagement. J Crohns Colitis. 2012;6:991—1030.
- Dignass A, Akbar A, Hart A, et al. Safety and efficacy of granulocyte/monocyte apheresis in steroid-dependent active ulcerative colitis with insufficient response or intolerance to immunosuppressants and/or biologics [the ART trial]: 12-week interim results. J Crohns Colitis. 2016;10:812–820.
- Dittrich K, Richter M, Rascher W, et al. Leukocytapheresis in a girl with severe ulcerative colitis refractory to corticosteroids, infliximab, and cyclosporine A. Inflamm Bowel Dis. 2008;14:1466–1467.
Contact UsFor more information
Contact Us